- 产品特性
- 相关资料
- Q&A
- 参考文献
![]() 细胞培养基质 层粘连蛋白511
细胞培养基质 层粘连蛋白511
iMatrix-511
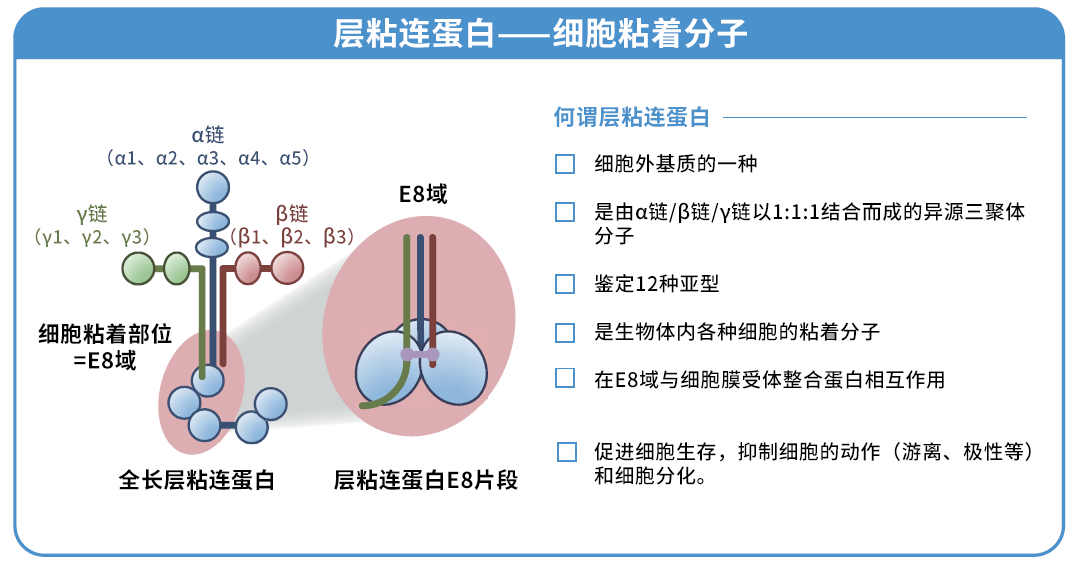
◆什么是层粘连蛋白511?
层粘连蛋白是存在于动物基底膜的一种细胞外基质,已知其与细胞粘附和增殖息息相关。本产品是与层粘连蛋白 511-E8 片段有同一序列的重组蛋白,是可以促进各种细胞粘附和伸展的培养基质。
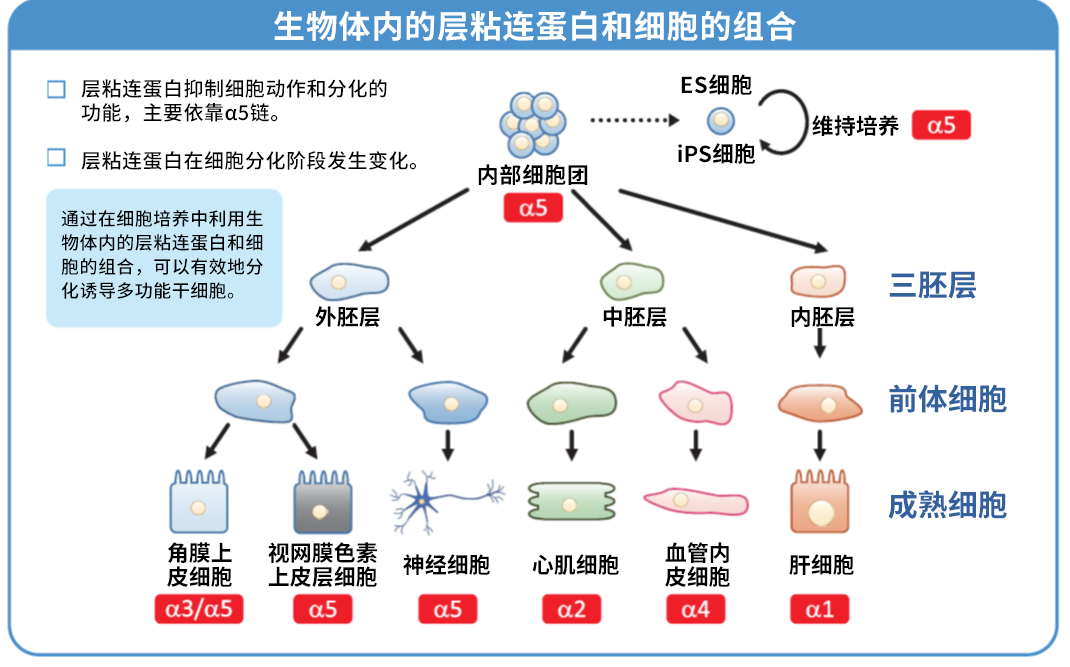
大阪大学和京都大学共同研究开发,本产品已证明在操作难度非常大的人 iPS 细胞和人 ES 细胞培养中,也可以安全且高效地进行细胞培养。
● 细胞培养的准备非常简单!
● 可用于多种类型的细胞培养!
● 无论分离细胞的状态如何,可实现细胞的高生存率和细胞增殖的高效性!
● 重组蛋白(CHO-S细胞来源),所以混入杂质的危险性低!
● 溶液型试剂,无需溶解,稀释后可直接使用

层粘连蛋白511 是由 α5 链、β1 链和 γ1 链组成的层粘连蛋白。层粘连蛋白 511-E8 是层粘连蛋白片段,但其具有与层粘连蛋白全长分子相同的 α6β1 整合蛋白连接功能。
本产品是 Nippi 根据大阪大学和京都大学的专利技术生产贩卖的。
◆使用方法
用 PBS(-)稀释本产品,按照 0.1~0.5 μg/cm2 加入细胞培养器皿中。
※由于细胞种类和细胞株的不同、使用的培养基种类不同,添加的最适剂量会有差异,初次使用时,按照 0.5 μg/cm2 添加培养容器中,逐渐
※调整至最佳使用浓度。
↓
室温下孵育 3 小时,然后去掉溶液。
↓
添加细胞和培养液,进行细胞培养。
● 培养 ES/iPS 细胞时,可进行无饲养层和单细胞继代培养
◆使用案例
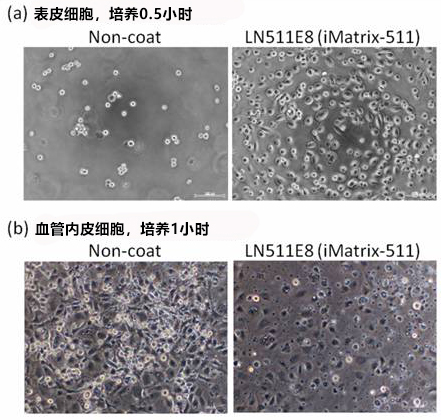
使用本产品对表皮细胞培养 0.5 小时,对血管内皮细胞培养 1 小时。
(a) 表皮细胞,培养 0.5 小时
左(无涂层):大部分细胞未贴壁。
右(iMatrix):多数细胞贴壁并成伸展状态。
(b) 血管内皮细胞,培养 1 小时
左(无涂层):有贴壁的细胞,但大部分多为圆形。
右(iMatrix):较少观察到圆形细胞,几乎所有的细胞表现出了很好的伸展性。
实验人员: (株) Nippi BioMatrix 研究所 藤崎
◆iMatrix-511 与 iMatrix-511 silk 的区别
|
iMatrix-511 |
iMatrix-511 silk |
|
|
生产系统 |
转基因 CHO-S 细胞 |
转基因蚕生产系统 |
|
提纯材料 |
CHO-S 细胞培养上清 |
蚕蛹蛋白 |
|
产品等级 |
实验研究用* *有临床用级别 |
实验研究用 |
|
导入基因 |
人层粘连蛋白 511-E8 片段 |
|
|
纯度 |
95% 以上 |
|
|
浓度 |
0.5 mg/mL |
|
|
解离常数 |
10 nM 以下 |
|
|
使用期限 |
生产后 2 年内 |
|
|
iPS 细胞培养能力 |
添加 0.5 μg/cm2 到培养容器中,可用于 iPS 细胞的维持培养 |
|
◆产品列表
|
产品编号 |
生产商编号 |
产品名称 |
包装 |
|
385-07361 |
892011 |
iMatrix-511 solution (0.5 mg/mL) 层粘连蛋白511-E8片段, 溶液(0.5 mg/mL) |
175 µg×2(350 µL×2) |
|
381-07363 |
892012 |
175 µg×6(350 µL×6) |
※ 本页面产品仅供研究用,研究以外不可使用。
点击此处,选择页面中的”iMatrix™ Calculator“计算实验中 iMatrix-511 使用量
点击此处进一步获取文献应用实例
点击此处下载产品宣传页
iMatrix-511,iMatrix-511 silk
Q1. 产品是以什么样的状态销售的?
A1. 产品是以液态销售的。一支试管里密封装有 0.5 mg/mL 浓度的 175 μg 的细胞培养外基质(层粘连蛋白 511-E8 片段)。
※iMatrix-511 的冻干产品已于2015年3月停止生产。
Q2. 产品的存储条件和有效期是什么?
A2. 产品的储存条件为,冷藏保存在 2-15°C。(推荐 4°C)
A2. 产品的有效期,请参考下表。
|
产品 |
保质期 |
|
iMatrix-511 |
自生产后两年内 |
|
iMatrix-511silk |
自生产后两年内 |
|
iMatrix-511MG |
自生产后两年内 |
※具体的有效期详见产品外包装。
Q3. 可以冷冻保存吗?
A3. 不可以冷冻保存。
Q4. 产品的纯度是多少?
A4. 纯度为 95% 以上。
Q5. 培养 hES/hiPS 细胞时使用什么培养基最好?
A5. 宫崎等的论文(Nature communications, 3(1236), 1-10, 2012)、(Scientific Reports, 7, 41165, 2017)中,使用了以下的培养基。
・mTeSR1,TeSR2,TeSR-E8 (STEMCELL Technologies)
・Stem Pro hESC SFM (Thermo Fisher Scientific)
・StemFit AK03 (Ajinomoto)
中川等的论文(Scientific Reports, 4(3594), 1-7, 2014)中使用了以下的培养基。
・StemFit (Ajinomoto)
文献中使用的培养基都出现了良好的结果。
Q6. 培养 iPS 细胞时,最佳的涂层浓度是多少?
A6. 最佳的涂层浓度根据细胞株的不同也会有所不同。
A6. 最初请从浓度 0.5 μg/cm2 开始尝试,请根据您使用的细胞株在浓度 0.1~1.5 μg/ cm2 之间考虑。
A6. 另外,还有文献报道了新的不以涂层包被的添加法。
A6. Miyazaki et al. Scientific Reports, 7, 41165, (2017)
Q7. 请教我使用 iMatrix-511 时,培养 iPS 细胞的步骤。
A7. 使用 iMatrix-511 时,ES/iPS 细胞的扩增培养步骤,传代操作,请参考以下的链接。
(扩增培养步骤 )
(传代操作的视频)
Q8. 培养 iPS 细胞时,需要 Rock Inhibitor(Y-27632)吗?
A8. 在中川等的论文(Scientific Reports, 4(3594), 2014)中,介绍了只有在传代时添加 Rock Inhibitor,更换培养基的时候不需使用。
Q9. 培养 iPS 细胞时可以单细胞传代吗?
A9. 可以。
※使用 iMatrix-511 时,ES/iPS 细胞的扩增培养步骤,传代操作,请参考以下的链接。
(扩增培养步骤 )
(传代操作的视频)
也可以参考中川等的论文(Scientific Reports, 4(3594), 2014)。
Q10. 传代时使用什么细胞分离液?
A10. 可使用胰蛋白酶。
※使用 iMatrix-511 时,ES/iPS 细胞的扩增培养步骤,传代操作,请参考以下的链接。
(扩增培养步骤)
(传代操作的视频)
也可以参考中川等的论文(Scientific Reports, 4(3594), 2014)。
Q11. 可以使用小鼠的 iPS 细胞吗?
A11. 由于没有小鼠 iPS 细胞的培养数据,所以无法回答。
Q12. 这个产品与基质胶有什么不同?
A12. 基质胶含有小鼠EHS肉瘤来源的层粘连蛋白-111。另外还含有层粘连蛋白以外的分子。
A12. iMatrix-511 是将在 CHO-S 细胞中表达的层粘连蛋白 511-E8 片段高度纯化后的重组蛋白。
A12.iMatrix-511silk 是从蚕结的茧中高纯度纯化层粘连蛋白 511-E8 片段的重组蛋白。
A12.已知人ES细胞和 iPS 细胞是通过细胞膜受体(特别是 α6β1整联蛋白)粘附于层粘连蛋白-511。
A12.已知人ES细胞和 iPS 细胞对层粘连蛋白-511 具有高粘附活性。这使得用 iMatrix-511/iMatrix-511silk 可以使 iPS 细胞在单细胞状态下传代。
A12.在宫崎等的论文中(Nature communications, 3(1236), 1-10, 201),5 次传代后(30 天后)的细胞数扩增效率约为基质胶的 200 倍。
参考文献
|
分类 |
文献信息 |
主题 |
|
人多能干细胞(hPSC)的确立 |
Miyazaki et al. Nat. Commun.3:1236, (2012) |
证实用作hPSC的培养基质的有效性 |
|
Nakagawa et al. Sci. Rep. 4:3594, (2014) |
确立医疗等级的hPSC |
|
|
Takashima et al. Cell.158(6):1254-69, (2014) |
促进向hPSC的基质状态的转移 |
|
|
Miyazaki et al. Sci. Rep.7:41165, (2017) |
采用无需涂层操作的添加法培养hPSC |
|
|
Sekine et al. Stem Cell Res.24:40-43, (2017) |
确立疾病特异性的hPSC |
|
|
Tan et al. Stem Cell Res. 24:12-15, (2017) |
||
|
Ishida et al. Sci. Rep. 8(1), 310, (2018) |
利用hPSC的基因编辑建立遗传性疾病模型 |
|
|
Kim et al. Nature Communications, 9(1), 939, (2018) |
||
|
Sakai-Takemura et al. Sci. Rep, 8, 6555, (2018) |
悬浮培养由hPSC分化的肌肉前体细胞 |
|
|
由hPSC分化衍生的细胞 |
Doi et al. Stem Cell Reports. 2(3):337-50, (2014) |
多巴胺产生神经元 |
|
Ishikawa et al. Hum. Mol. Genet.25(23): 5188-5197, (2016) |
||
|
Nishimura et al. Stem Cell Reports.6(4): 511-524, (2016) |
||
|
Samata et al. Nat. Commun. 7:13097, (2016) |
||
|
Kikuchi et al. Nature. 548(7669):592-596, (2017) |
||
|
Morizane et al. Nat. Commun.8(1):385, (2017) |
||
|
Kikuchi et al. J. Neurosci. Res.95(9):1829-37, (2017) |
||
|
Goparaju et al. Sci. Rep. 7:42367, (2017) |
运动神经元 |
|
|
Burridge et al. Nat. Methods.11(8):855-60, (2014) |
心肌细胞 |
|
|
Sougawa et al. Sci. Rep,8(1), 3726, (2018) |
||
|
Yamauchi et al. BBRC, 495(1), 1278-1284, (2018) |
心室肌细胞 |
|
|
Akiyama et al. Sci. Rep, 8(1), 1189, (2018) |
骨骼肌细胞 |
|
|
Saito et al. Stem Cell Res Ther, 9(1), 12, (2018) |
成骨细胞 |
|
|
Uchimura et al. Stem cell research, 25, 98-106, (2017) |
成肌细胞 |
|
|
Hayashi et al. Nature.531(7594):376-80, (2016) |
视觉细胞 |
|
|
Hayashi et al. Nat. Protoc.12(4):683-696, (2017) |
角膜上皮细胞 |
|
|
Takayama et al. BBRC. 474(1):91-96, (2016) |
胆管上皮细胞 |
|
|
Takayama et al. Hepatol Commun,1(10), 1058-1069, (2017) |
肝实质细胞 |
|
|
Takayama et al. Biomaterials, (2018) |
||
|
Takebe et al. Cell Reports, 21(10), 2661-2670, (2017) |
肝细胞 |
|
|
Tan et al. Stem Cell Reports, 11:1-11, (2018) |
||
|
Camp et al. Nature. 546(7659):533-38, (2017) |
定形内胚层细胞 |
|
|
Zhang et al. Stem Cell Reports, 10(2), 1–14, (2018) |
后内胚层前体细胞 |
|
|
Tanigawa et al. Cell reports, 15(4), 801-813, (2016) |
肾单位前体细胞(胎肾细胞) |
|
|
Musah et al. Nat.Biomed.Eng.1:0069, (2017) |
肾小球上皮细胞 |
|
|
Musah et al. Nature protocols,13(7):1662, (2018) |
||
|
Mae et al. BBRC, 495(1), 954-961, (2018) |
输尿管芽组织 |
|
|
Oshima et al. BBRC, 497(2), 719-725, (2018) |
血细胞・血管内皮常见前体细胞 |
|
|
Taguchi et al. Cell Stem Cell, 21, (2017) |
*培养hPSC用于分化肾单位前体细胞(胎肾细胞) |
|
|
Kawamura et al. Stem Cell Reports.6(3):312-20,(2016) |
*培养hPSC用于分化心肌细胞 |
|
|
Sasaki et al. Cell Stem Cell.17(2):178-94, (2015) |
*培养hPSC用于分化生殖细胞 |
|
|
Kojima et al. Cell Stem Cell.21(4):517-532, (2017) |
||
|
Furuta et al. PLoS One. 9(12):e112291, (2014) |
*培养hPSC用于分化间充质细胞 |
|
|
人原代细胞的培养 |
Okumura et al. Invest. Ophth. Vis. Sci.56(5):2933-42, (2015) |
人角膜内皮细胞 |
|
Hongo et al. Invest. Ophth. Vis. Sci.58(9):3325-34, (2017) |
||
|
Polisetti et al. Sci. Rep.7(1):5152, (2017) |
人角膜边缘上皮前体细胞 |
|
|
Ishii et al. Stem Cell Reports, 10, 1-15, (2018) |
卫星细胞 |
|
|
层粘连蛋白-整合素相互作用的分子机制 |
Ido et al. J. Biol. Chem. 282(15): 11144-54, (2007) |
|
|
Ido et al. J. Biol. Chem.283(42): 28149-57, (2008) |
||
|
Taniguchi et al. J. Biol. Chem. 284(12): 7820-31, (2009) |
||
|
Taniguchi et al. BBRC.487(3): 525-531, (2017) |
||
|
Takizawa et al. Sci Adv.3(9) :e1701497, (2017) |
英文论文
|
[1] |
Ayabe, H., Anada, T., Kamoya, T., Sato, T., Kimura, M., Yoshizawa, E., Kikuchi, Shunyuu., Ueno, Yasuharu., Sekine, keisuke., J. Gray Camp., Treutlein, B., Ferguson, Autumn., Suzuki, Osamu., Takede, Takanori.. Optimal Hypoxia Regulates Human iPSC-Derived Liver Bud Differentiation through Intercellular TGFB Signaling. Stem Cell Reports, 11, 1-11, (2018)
|
|
[2] |
Musah, S., Dimitrakakis, N., Camacho, D. M., Church, G. M., Ingber, D. E.. Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a lomerulus Chip. Nature protocols, 13(7), 1662, (2018)
|
|
[3] |
Ishii, K., Sakurai, H., Suzuki, N., Mabuchi, Y., Sekiya, I., Sekiguchi, K., Akazawa, C.. Recapitulation of Extracellular LAMININ Environment Maintains Stemness of Satellite Cells In Vitro. Stem Cell Reports, 10, 1-15, (2018)
|
|
[4] |
Ishida, K., Xu, H., Sasakawa, N., Lung, M. S. Y., Kudryashev, J. A., Gee, P., & Hotta, A.. Site-specific randomization of the endogenous genome by a regulatable CRISPR-Cas9 piggyBac system in human cells. Scientific Reports, 8(1), 310, (2018)
|
|
[5] |
Takayama, K., Hagihara, Y., Toba, Y., Sekiguchi, K., Sakurai, F., Mizuguchi, H.. Enrichment of high- functioning human iPS cell-derived hepatocyte-like cells for pharmaceutical research. Biomaterials, (2018)
|
|
[6] |
Akiyama, T., Sato, S., Chikazawa-Nohtomi, N., Soma, A., Kimura, H., Wakabayashi, S., Ko, S.B., Ko, M. S.. Efficient differentiation of human pluripotent stem cells into skeletal muscle cells by combining RNA-based MYOD1-expression and POU5F1-silencing. Scientific Reports, 8(1), 1189, (2018)
|
|
[7] |
Saito, A., Ooki, A., Nakamura, T., Onodera, S., Hayashi, K., Hasegawa, D., Okudaira,T., Watanabe, K., Kato, H., Onda, T., Watanabe, A., Kosaki, K., Nishimura, K., Ohtaka, Manami., Nakanishi, M., Sakamoto, T., Yamaguchi, A., Sueishi, K., Azuma, T.. Targeted reversion of induced pluripotent stem cells from patients with human cleidocranial dysplasia improves bone regeneration in a rat calvarial bone defect model. Stem Cell Research & Therapy, 9(1), 12, (2018)
|
|
[8] |
Yamauchi, K., Li, J., Morikawa, K., Liu, L., Shirayoshi, Y., Nakatsuji, N., Elliott, A. D., Hisatome, I., Suemori, H..Isolation and characterization of ventricular-like cells derived from NKX2-5 eGFP/w and MLC2v mCherry/w double knock-in human pluripotent stem cells. Biochemical and Biophysical Research Communications, 495(1), 1278-1284, (2018)
|
|
[9] |
Mae, S., Ryosaka, M., Toyoda, T., Matsuse, K., Oshima, Y., Tsujimoto, H., Okumura, S., Shibasaki, A., Osafune, K.. Generation of branching ureteric bud tissues from human pluripotent stem cells. Biochemical and biophysical research communications, 495(1), 954-961, (2018)
|
|
[10] |
Kagihiro, M., Fukumori, K., Aoki, T., Ungkulpasvich, U., Mizutani, M., Viravaidya-Pasuwat, K.,& Kino-oka, M.. Kinetic analysis of cell decay during the filling process: Application to lot size determination in manufacturing systems for human induced pluripotent and mesenchymal stem cells. Biochemical Engineering Journal, 131, 31-38, (2018)
|
|
[11] |
Zhang, R. R., Koido, M., Tadokoro, T., Ouchi, R., Matsuno, T., Ueno, Y., Sekine, K., Takebe, T., Taniguchi, H.. Human iPSC-Derived Posterior Gut Progenitors Are Expandable and Capable of Forming Gut and Liver Organoids. Stem Cell Reports, 10(2), 1?14, (2018)
|
|
[12] |
Oshima, K., Saiki, N., Tanaka, M., Imamura, H., Niwa, A., Tanimura, A., Nagahashi, A., Hirayama, A., Okitac, K., Hotta, A., Kitayama, S., Osawa, M., Kaneko, S., Watanabe, A., Asaka, I., Fujibuchi, W., Imai, K., Yabe, H., Kamachi, Y., Hara, J., Kojima, S., Tomita, M., Soga, T., Noma, T., Nonoyama, S., Nakahata, T., Saito, MK.. Human AK2 links intracellular bioenergetic redistribution to the fate of hematopoietic progenitors. Biochemical and Biophysical Research Communications, 497(2), 719- 725, (2018)
|
|
[13] |
Sougawa, N., Miyagawa, S., Fukushima, S., Kawamura, A., Yokoyama, J., Ito, E., Harada, A., Okimoto, K., Mochisuki-Oda, N., Saito, A., Sawa, Y.. Immunologic targeting of CD30 eliminates tumourigenic human pluripotent stem cells, allowing safer clinical application of hiPSC-based cell therapy. Scientific Reports, 8(1), 3726, (2018)
|
|
[14] |
Yasuda, S. Y., Ikeda, T., Shahsavarani, H., Yoshida, N., Nayer, B., Hino, M., Vartak-Sharma, N., Suemori, H., Hasegawa, K.. Chemically defined and growth-factor-free culture system for the expansion and derivation of human pluripotent stem cells. Nature Biomedical Engineering, 2(3), 173, (2018)
|
|
[15] |
Kim, S. I., Matsumoto, T., Kagawa, H., Nakamura, M., Hirohata, R., Ueno, A., Ohishi, M., Sakuma, T., Soga, T., Yamamoto, T., Woltjen, K.. Microhomology-assisted scarless genome editing in human iPSCs. Nature Communications, 9(1), 939, (2018)
|
|
[16] |
Hayashi, R., Ishikawa, Y., Katori, R., Sasamoto, Y., Taniwaki, Y., Takayanagi, Tsujikawa, M., Sekiguchi, K., Quantock, A. J., Nishida, K. . Coordinated generation of multiple ocular-like cell lineages and fabrication of functional corneal epithelial cell sheets from human iPS cells. Nature Protocols, 12(4), 683-696, (2017)
|
|
[17] |
Kikuchi, T., Morizane, A., Okita, K., Nakagawa, M., Yamakado, H., Inoue, H., Takahashi, R., Takahashi, J. . Idiopathic Parkinson's disease patient‐derived induced pluripotent stem cells function as midbrain dopaminergic neurons in rodent brains. Journal of Neuroscience Research, 95(9),1829-37, (2017)
|
|
[18] |
Miyazaki, T., Isobe, T., Nakatsuji, N., & Suemori, H. . Efficient Adhesion Culture of Human Pluripotent Stem Cells Using Laminin Fragments in an Uncoated Manner. Scientific Reports, 7 (41165), 1-8, (2017)
|
|
[19] |
Goparaju, S. K., Kohda, K., Ibata, K., Soma, A., Nakatake, Y., Akiyama, T., Wakabayashi, S., Matsushita, M., Sakota, M., Kimura, H., Yuzaki, M., Shigeru B. H. Ko & Minoru S. H. Ko. . Rapid differentiation of human pluripotent stem cells into functional neurons by mRNAs encoding transcription factors. Scientific Reports, 7, 42367, (2017)
|
|
[20] |
Musah, S., Mammoto, A., Ferrante, C. T., Jeanty, S.S., Hirano-Kobayashi, M., Mammoto, T., Roberts, K., Chung, S., Novak, R., Ingram, M., Fatanat-Didar, T., Koshy, S., Weaver, C. J., Church, M. G., Ingber, F. D. . Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nature Biomedical Engineering, 1 (0069), (2017)
|
|
[21] |
Camp, J. G., Sekine, K., Gerber, T., Loeffler-Wirth, H., Binder, H., Gac, M., Kanton, S., Kageyama, J., Damm, G., Seehofer, D., Belicova, L., Barsacchi, M., Barsacchi, R., Okuda, R., Yoshizawa, E., Kimura, M., Ayabe, H., Taniguchi, H., Takebe, T., & Belicova, L.. Multilineage communication regulates human liver bud development from pluripotency. Nature, 546, 533-538, (2017)
|
|
[22] |
Polisetti, N., Sorokin, L., Okumura, N., Koizumi, N., Kinoshita, S., Kruse, F. E., and Schlotzer- Schrehardt, U. Laminin-511 and-521-based matrices for efficient ex vivo-expansion of human limbal epithelial progenitor cells. Scientific Reports, 7, 5152, (2017)
|
|
[23] |
Hongo, A., Okumura, N., Nakahara, M., Kay, E. P., & Koizumi, N.. The Effect of a p38 Mitogen- Activated Protein Kinase Inhibitor on Cellular Senescence of Cultivated Human Corneal Endothelial CellsEffect of a p38 MAPK Inhibitor on Corneal Endothelial Cells. Investigative Ophthalmology & Visual Science, 58(9), 3325-3334, (2017)
|
|
[24] |
Taniguchi, Y., Li, S., Takizawa, M., Oonishi, E., Toga, J., Yagi, E., & Sekiguchi, K. Probing the acidic residue within the integrin binding site of laminin-511 that interacts with the metal ion- dependent adhesion site of α6β1 integrin. Biochemical and Biophysical Research Communications, 487(3), 525-531, (2017)
|
|
[25] |
Sekine, S. I., Kondo, T., Murakami, N., Imamura, K., Enami, T., Shibukawa, R., Tsukita, K., Funayama, M., Inden, M., Kurita, H., Hozumi, I., Inoue, H.. Induced pluripotent stem cells derived from a patient with familial idiopathic basal ganglia calcification (IBGC) caused by a variant in SLC20A2 gene. Stem Cell Research, (2017)
|
|
[26] |
Tan, G. W., Kondo, T., Murakami, N., Imamura, K., Enami, T., Tsukita, K., Shibukawa, R., Funayama, M., Matsumoto, R., Ikeda, I., Takahashi, R., Inoue, H.. Induced pluripotent stem cells derived from an autosomal dominant lateral temporal epilepsy (ADLTE) patient carrying S473L mutation in leucine-rich glioma inactivated 1 (LGI1). Stem Cell Research, (2017)
|
|
[27] |
Sato-Nishiuchi, R., Li, S., Ebisu, F., Sekiguchi, K.. Recombinant laminin fragments endowed with collagen-binding activity: A tool for conferring laminin-like cell-adhesive activity to collagen matrices. Matrix Biology, (2017)
|
|
[28] |
Kikuchi, T., Morizane, A., Doi, D., Magotani, H., Onoe, H., Hayashi, T., Mizuma, H., Takara, S., Takahashi, R., Inoue, H., Morita, S., Yamamoto, M., Okita, K., Nakagawa, M., Parmar, M., Takahashi, J.. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature, 548, 592-596, (2017)
|
|
[29] |
Takizawa, M., Arimori, T., Taniguchi, Y., Kitago, Y., Yamashita, E., Takagi, J., Sekiguchi, K.. Mechanistic basis for the recognition of laminin-511 by α6β1 integrin. Science Advances, 3(9), e1701497, (2017)
|
|
[30] |
Morizane, A., Kikuchi, T., Hayashi, T., Mizuma, H., Takara, S., Doi, H., Mawatari, A., Glasser, M.F., Shiina, T., Ishigaki, H., Itoh, Y., Okita, K., Yamasaki, E., Doi, D., Onoe, H., Ogasawara, K., Yamanaka, S., and Takahashi, J. . MHC matching improves engraftment of iPSC-derived neurons in non- human primates. Nature Communications, 8(1), 385, (2017)
|
|
[31] |
Kikuchi, T., Morizane, A., Doi, D., Magotani, H., Onoe, H., Hayashi, T., Mizuma, H., Takara, S., Takahashi, R., Inoue, H., Morita, S., Yamamoto, M., Okita, K., Nakagawa, M., Parmar, M., Takahashi, J. . human ips cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature, 548(7669), 592-596, (2017)
|
|
[32] |
Kojima, Y., Sasaki, K., Yokobayashi, S., Sakai, Y., Nakamura, T., Yabuta, Y., Nakaki, F., Nagaoka, S., Woltjen, K., Hotta, A., Yamamoto, T., Saitou, M.. Evolutionarily Distinctive Transcriptional and Signaling Programs Drive Human Germ Cell Lineage Specification from Pluripotent Stem Cells. Cell Stem Cell, 21(4), 517-532.e5, (2017)
|
|
[33] |
Taguchi, A., & Nishinakamura, R.. Higher-Order Kidney Organogenesis from Pluripotent Stem Cells. Cell Stem Cell, 21. (2017)
|
|
[34] |
Takebe, T., Sekine, K., Kimura, M., Yoshizawa, E., Ayano, S., Koido, M., Funayama, S., Nakanishi, N., Hisai, T., Kobayashi, T., Kasai, T., Kitada, R., Mori, A., Ayabe, H., Ejiri, Y., Amimoto, N., Yamazaki, Y., Ogawa, S., Ishikawa, M., Kiyota, Y., Sato, Y., Nozawa, K., Okamoto, S., Ueno, Y., Kasai, T.. Massive and Reproducible Production of Liver Buds Entirely from Human Pluripotent Stem Cells. Cell Reports, 21(10), 2661-2670, (2017)
|
|
[35] |
Uchimura, T., Otomo, J., Sato, M., Sakurai, H.. A human iPS cell myogenic differentiation system permitting high-throughput drug screening. Stem cell research, 25, 98-106, (2017)
|
|
[36] |
Sougawa, N., Miyagawa, S., Fukushima, S., Saito, A., Yokoyama, J., Kitahara, M., Harada, A., Sato- Nishiuchi, R., Sekiguchi, K., Sawa, Y.. Novel Stem Cell Niches Laminin 511 Promotes Functional Angiogenesis Through Enhanced Stem Cell Homing by Modulating" Stem Cell Beds" in the Failed Heart.Circulation, 136(1), A15587, (2017)
|
|
[37] |
Samata, B., Doi, D., Nishimura, K., Kikuchi, T., Watanabe, A., Sakamoto, Y., Kakuta, J., Ono, Y.,& Takahashi, J.. Purification of functional human ES and iPSC-derived midbrain dopaminergic progenitors using LRTM1. Nature Communications, 7(13097), 1-11, (2016)
|
|
[38] |
Hayashi, R., Ishikawa, Y., Sasamoto, Y., Katori, R., Nomura, N., Ichikawa, T., Araki, S., Soma, T., Kawasaki, S., Sekiguchi, K., Tsujikawa, M., Nishida, K., & Quantock, A. J.. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature, 531(7594), 376-380, (2016)
|
|
[39] |
Matsuno, K., Mae, S. I., Okada, C., Nakamura, M., Watanabe, A., Toyoda, T., Uchida, E., Osafune, K.. Redefining definitive endoderm subtypes by robust induction of human induced pluripotent stem cells.Differentiation; research in biological diversity, (2016)
|
|
[40] |
Nishimura, K., Doi, D., Samata, B., Murayama, S., Tahara, T., Onoe, H., & Takahashi, J.. Estradiol Facilitates Functional Integration of iPSC-Derived Dopaminergic Neurons into Striatal Neuronal Circuits via Activation of Integrin α5β1. Stem cell reports, 6(4), 511-524, (2016)
|
|
[41] |
Takayama, K., Mitani, S., Nagamoto, Y., Sakurai, F., Tachibana, M., Taniguchi, Y., Sekiguchi,K., Mizuguchi, H.. Laminin 411 and 511 promote the cholangiocyte differentiation of human induced pluripotent stem cells. Biochemical and biophysical research communications, 474(1), 91-96, (2016)
|
|
[42] |
Kawamura, T., Miyagawa, S., Fukushima, S., Maeda, A., Kashiyama, N., Kawamura, A., Miki, K., Okita, K., Yoshida, Y., Shiina, T., Ogasawara, K., Miyagawa, S., Toda, K., Okuyama, H., Sawa,Y.. Cardiomyocytes derived from MHC-homozygous induced pluripotent stem cells exhibit reduced allogeneic immunogenicity in MHC-matched non-human primates. Stem cell reports, 6(3), 312- 320, (2016).
|
|
[43] |
Tanigawa, S., Taguchi, A., Sharma, N., Perantoni, A. O., & Nishinakamura, R.. Selective in vitro propagation of nephron progenitors derived from embryos and pluripotent stem cells. Cell reports, 15(4), 801-813, (2016)
|
|
[44] |
Okumura, N., Kakutani, K., Numata, R., Nakahara, M., Schlotzer-Schrehardt, U., Kruse, F., Kinoshita. K., Koizumi, N.. Laminin-511 and-521 Enable Efficient In Vitro Expansion of Human Corneal Endothelial CellsLaminin-511 and-521 Enable Expansion of HCECs. Investigative ophthalmology & visual science, 56(5), 2933-2942, (2015)
|
|
[45] |
Sasaki, K., Yokobayashi, S., Nakamura, T., Okamoto, I., Yabuta, Y., Kurimoto, K., Ohta, H., Moritoki, Y., Iwatani, C., Tsuchiya, H., Nakamura, S., Sekiguchi, K., Sakuma, T., Yamamoto, T., Mori, T., Woltjen, K., Nakagawa, M., Yamamoto, T., Takahashi, K., Yamanaka, S., Saitou, M.. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell stem cell, 17(2), 178-194, (2015)
|
|
[46] |
Nakagawa, M., Taniguchi, Y., Senda, S., Takizawa, N., Ichisaka, T., Asano, K., Morizane, A., Doi, D., Takahashi, J., Nishizawa, M., Yoshida, Y., Toyoda, T., Osafune, K., Sekiguchi, K., & Yamanaka, S. . A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Scientific reports, 4(3594), 1-7, (2014)
|
|
[47] |
Doi, D., Samata, B., Katsukawa, M., Kikuchi, T., Morizane, A., Ono, Y., Sekiguchi, K., Nakagawa, M., Parmar, M., Takahashi, J.. Isolation of human induced pluripotent stem cell-derived dopaminergic progenitors by cell sorting for successful transplantation. Stem cell reports, 2(3), 337-350, (2014)
|
|
[48] |
Takashima, Y., Guo, G., Loos, R., Nichols, J., Ficz, G., Krueger, F., Oxley, D., Santos, F., Clarke, J., Mansfield, W., Reik, W., Bertone, P., Smith, A.. Resetting transcription factor control circuitry toward ground-state pluripotency in human. Cell, 158(6), 1254-1269, (2014)
|
|
[49] |
Fukuta, M., Nakai, Y., Kirino, K., Nakagawa, M., Sekiguchi, K., Nagata, S., Matsumoto, Y., Yamamoto, T., Umeda, K., Heike, T., Okumura, N., Koizumi, N., Sato, T., Nakahata, T., Saito, M., Otsuka, T., Kinoshita, S., Ueno, M., Ikeya, M., Toguchida, J. . Derivation of mesenchymal stromal cells from pluripotent stem cells through a neural crest lineage using small molecule compounds with defined media. PloS one, 9(12), e112291, (2014)
|
|
[50] |
Burridge, P. W., Matsa, E., Shukla, P., Lin, Z. C., Churko, J. M., Ebert, A. D., Lan, F., Diecke, S., Huber, B., Mordwinkin, N. M., Plews, J. R., Abilez, O. J., Cui, B., Gold, J. D., & Wu, J. C. . Chemically defined generation of human cardiomyocytes. Nature methods, 11(8), 855-860, (2014)
|
|
[51] |
Miyazaki, T., Futaki, S., Suemori, H., Taniguchi, Y., Yamada, M., Kawasaki, M., Hayashi, M., Kumagai, H., Nakatsuji, N., Sekiguchi, K., & Kawase, E. . Laminin E8 fragment support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nature communications, 3(1236), 1-10, (2012)
|
|
[52] |
Taniguchi, Y., Ido, H., Sanzen, N., Hayashi, M., Sato-Nishiuchi, R., Futaki, S., & Sekiguchi, K. . The C-terminal region of laminin β chains modulates the integrin binding affinities of laminins. Journal of Biological Chemistry, 284(12), 7820-7831, (2009)
|
|
[53] |
Ido, H., Nakamura, A., Kobayashi, R., Ito, S., Li, S., Futaki, S., & Sekiguchi, K. . The requirement of the glutamic acid residue at the third position from the carboxyl termini of the laminin γ chains in integrin binding by laminins. Journal of Biological Chemistry, 282(15), 11144-11154, (2007) |
| 产品编号 | 产品名称 | 产品规格 | 产品等级 |
