上海金畔生物科技有限公司代理New England Biolabs(NEB)酶试剂全线产品,欢迎访问官网了解更多产品信息和订购。
产品信息
ARCA is incorporated into mRNA exclusively in the correct orientation, generating capped mRNA that is more efficiently translated. Standard cap analogs can be incorporated in either direction resulting in only 50% of capped mRNA that is functional in protein translation.
- 产品类别:
- RNA Capping,
- RNA Synthesis In vitro Transcription (IVT)
-
试剂盒组成
本产品提供以下试剂或组分:
NEB # 名称 组分货号 储存温度 数量 浓度 -
E2060S -20 Poly(A) Polymerase Reaction Buffer B0276SVIAL -20 1 x 1.5 ml 10 X T7 RNA Polymerase Mix M0255AAVIAL -20 1 x 0.04 ml Not Applicable ARCA/NTP Mix N2053AVIAL -20 1 x 0.2 ml Not Applicable DNase I (RNase-free) M0303AAVIAL -20 1 x 0.04 ml 2,000 units/ml CLuc Control Template N0247AVIAL -20 1 x 20 µl 0.25 mg/ml LiCl Solution B2051AVIAL -20 1 x 1.4 ml Not Applicable E. coli Poly(A) Polymerase M0444AVIAL -20 1 x 0.1 ml Not Applicable Dithiothreitol (DTT) B1222AVIAL -20 1 x 0.5 ml 100 mM
-
-
特性和用法
需要但不提供的材料
- DNA template
- Thermocycler or 37°C incubator.
- Nuclease-free water
- Buffer- or water-saturated phenol:chloroform
- Ethanol
- 3 M Sodium acetate, pH 5.2
- 5 M Ammonium acetate
- Spin columns (see Monarch® RNA Cleanup Kits, NEB #T2040 or #T2050)
- Gels, running buffers and gel box
- Equipment for RNA analysis
-
相关产品
相关产品
- e2040-hiscribe-t7-high-yield-rna-synthesis-kit
- e2050-hiscribe-t7-quick-high-yield-rna-synthesis-kit
- e2065-hiscribe-t7-arca-mrna-kit
- 2X RNA 上样染料
- m0307-rnase-inhibitor-human-placenta
- 小鼠 RNase 抑制剂
- m0303-dnase-i-rnase-free
- Q5® 热启动超保真 DNA 聚合酶
- ssRNA Ladder
- 低分子量 ssRNA Ladder
- s1411-3-o-me-m7g5ppp5g-rna-cap-structure-analog
- s1405-m7g5ppp5a-rna-cap-structure-analog
- s1406-g5ppp5a-rna-cap-structure-analog
- s1407-g5ppp5g-rna-cap-structure-analog
- s1404-m7g5ppp5g-rna-cap-structure-analog
- m2080-vaccinia-capping-system
- mRNA 帽结构 2-O-甲基转移酶
- E. coli Poly(A) 聚合酶
- rNTP 混合液
- rNTP 套装
- Monarch® PCR & DNA 纯化试剂盒(5 μg)
- T2040 Monarch RNA Cleanup Kit 50 ug
-
注意事项
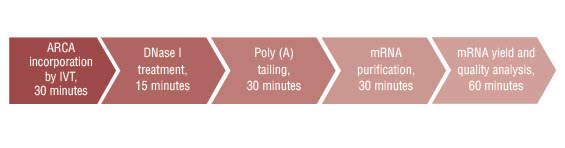
- 试剂盒所有组分都应贮存于 –20℃。试剂盒提供的试剂足够进行每次 20 μl 的 20 次反应。每次标准反应 1 μg 对照模板可合成高达 20 μg 的加帽 mRNA。Poly(A) 加尾和 LiCl 沉淀纯化后,可获得高达 25 μg 的加帽和加尾 mRNA。
操作说明、说明书 & 用法
-
操作说明
- Standard mRNA Synthesis (E2060)
- mRNA Synthesis with Modified Nucleotides (E2060)
- mRNA Purification (E2060)
- Evaluation of Reaction Products (E2060)
-
说明书
产品说明书包含产品使用的详细信息、产品配方和质控分析。- manualE2060
-
应用实例
- Scaling of High-Yield In vitro Transcription Reactions for Linear Increase of RNA Production
FAQs & 问题解决指南
-
FAQs
- HiScribe® T7 ARCA mRNA Kit (with tailing) What is the difference between the HiScribe T7 ARCA mRNA Kit (NEB #E2065) and the HiScribe T7 ARCA mRNA Kit (with Tailing)(NEB #E2060)?
- I currently use mMessage mMachine® T7 Ultra Transcription Kit, which mRNA synthesis kit from NEB should I use?
- I currently use MessageMAX™ T7 ARCA-capped Message Transcription Kit, which mRNA synthesis kit from NEB should I use?
- Can modified nucleotides be used with the HiScribe T7 ARCA mRNA kits?
- What is the difference between the HiScribe T7 ARCA mRNA kits and the HiScribe T7 High Yield RNA Synthesis Kit (E2040) and HiScribe T7 Quick RNA Synthesis Kit (E2050)?
- Can I use the Monarch RNA Cleanup Kits to cleanup my in vitro transcription (IVT) reaction?
- How can I improve on a low yield of RNA from the transcription reaction?
- Are modified nucleotides included in the kit?
- Do I need to add DTT to the reaction?
-
问题解决指南
- Control Reaction
The CLuc control template DNA is a linearized plasmid containing the Cypridina luciferase gene under the transcriptional control of the T7 promoter. The size of the runoff transcript is 1.6 kb. The control reaction should yield ≥ 15 μg RNA transcript in 30 minutes.If the control reaction is not working, there may be technical problems during reaction set up. Repeat the reaction by following the protocol carefully; take all precautions to avoid RNase contamination. Contact NEB for technical assistance.
The control plasmid sequence can be found here. The CLuc control template is generated by linearizing the plasmid with restriction enzyme Xba I.
- Low Yield of Full-length RNA
If the transcription reaction with your template generates full-length RNA, but the yield is significantly lower than expected, it is possible that contaminants in the DNA template are inhibiting the RNA polymerase, or the DNA concentration may be incorrect. Alternatively, additional purification of DNA template may be required. Phenol:chloroform extraction is recommended (see template DNA preparation section). - Low Yield of Short Transcript
High yields of short transcripts (< 0.3 kb) are achieved by extending incubation time and increasing the amount of template. Incubation of reactions up to 16 hours (overnight) or using up to 2 μg of template will help to achieve maximum yield. Alternatively, clean up the DNA template using a spin column
based method, Monarch PCR & DNA Cleanup Kit (5 μg), NEB #T1030. - RNA Transcript Smearing on Denaturing Gel
If the RNA appears degraded (e.g., smeared) on denaturing agarose or polyacrylamide gel, the DNA template is likely contaminated with RNase. DNA templates contaminated with RNase can affect the length and yield of RNA synthesized (a smear below the expected transcript length). We recommend evaluating the plasmid DNA template with the RNase Contamination assay Kit (NEB #E3320). If the plasmid DNA template is contaminated with RNase, perform phenol:chloroform extraction, then ethanol precipitate and dissolve the DNA in nuclease-free water. If the plasmid DNA template is contaminated with RNase, perform phenol:chloroform extraction, then ethanol precipitate and dissolve the DNA in nuclease-free water (see template DNA preparation section). - RNA Transcript of Larger Size than Expected
If the RNA transcript appears larger than expected on a denaturing gel, plasmid DNA may be incompletely digested. Even small amounts of undigested circular plasmid DNA can produce large amounts of long transcripts. Check template for complete digestion. If undigested plasmid is confirmed, repeat restriction enzyme digestion.Larger size bands may also be observed when the RNA transcript is not completely denatured due to the presence of strong secondary structures.
- RNA Transcript of Smaller Size than Expected
If denaturing gel analysis shows the presence of smaller bands than the expected size, it is most likely due to premature termination by the polymerase. Sequences with resemblance to T7 RNA Polymerase termination signals will cause premature termination. Incubating the transcription reaction at lower temperatures, for example at 30°C, may increase the proportion of full-length transcript, however the yield will be decreased. For GC rich templates, or templates with secondary structures, incubation at 42°C may improve yield of full-length transcript. - Tailing Length Control
A standard 30 min tailing reaction can add a poly(A) tail at least 150 nt in length to an average size mRNA generated from the IVT reaction. Short RNA may require longer incubation time for sufficient tailing. - No Tailing or Partial Tailing
3′ end of the mRNA must be exposed for efficient tailing. Because T7 RNA
Polymerase tends to generate 3′ end heterogeneity by adding extra bases, a
small percentage of the mRNA may adopt alternate structures which may not
be suitable for tailing. The following tips may help with successful tailing.
- Run the whole mRNA synthesis work flow without freezing the RNA
between steps. - To avoid preferential tailing, pre-incubate tailing mix at 37°C for 3 minutes
before adding Poly(A) Polymerase. Mix well immediately. - Tailing reaction should be at 37–40°C. Lower temperatures are not
recommended. - If still no tailing, redesign the DNA template with different sequences at
the 3′ end.
- Run the whole mRNA synthesis work flow without freezing the RNA
- mRNA not functional
- Verify the mRNA is intact, capped and tailed.
- Be sure the mRNA is clean, free from any inhibitors of downstream experiments.
- Follow instructions carefully with appropriate controls.
- Verify the DNA template has the correct sequence.
- Control Reaction
